Abstract
Background
We previously reported high response rates and durable remissions in patients (pts) with relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (ALL; Turtle, JCI 2016) and non-Hodgkin lymphoma (NHL; Turtle, Sci Transl Med 2016) treated with CD19-specific chimeric antigen receptor T (CD19 CAR-T) cells. In a subset of pts, we identified CD8+ T cell responses to epitopes in the murine CD19-binding single chain variable fragment (scFv) of the CAR that could limit CAR-T cell persistence and responses to subsequent infusions. In an effort to reduce the potential for immune CAR-T cell rejection, the murine CD19-binding scFv of the CAR was replaced with a fully human scFv linked to 4-1BB and CD3z signaling domains (JCAR021; Sommermeyer, Leukemia 2017). Here we report the initial clinical results of immunotherapy with JCAR021.
Methods
We initiated a phase I trial investigating lymphodepletion with cyclophosphamide 300 mg/m2/d and fludarabine 30 mg/m2/d for 3 days (Cy/Flu) followed by infusion of JCAR021 in pts with R/R ALL and aggressive NHL (NCT03103971). Pts were enrolled into 1 of 3 cohorts: high marrow burden ALL (HMB; > 5% blasts in bone marrow [BM] before lymphodepletion); low marrow burden ALL (LMB; ≤ 5% blasts in BM before lymphodepletion); and NHL. The starting dose was 7x104 JCAR021 cells/kg for the HMB ALL cohort, and 7x105 JCAR021 cells/kg in both the LMB ALL and NHL cohorts. Dose escalation/de-escalation follows a modified toxicity probability interval algorithm (Guo, Contemp Clin Trials 2017). Responses in the NHL cohort and in the HMB/LMB ALL cohorts were determined by the Lugano criteria (Cheson, JCO 2014) and the 2018 NCCN guidelines, respectively. Cytokine release syndrome (CRS) was graded according to consensus criteria (Lee, Blood 2014) and neurotoxicity was graded according to CTCAE v4.03.
Results
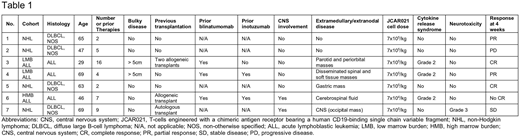
Pt characteristics are detailed in Table 1. As of June 15, 2018, 9 pts were enrolled on the trial. Two pts did not receive JCAR021: one pt was excluded after aggressive NHL was reclassified as indolent after pathology review and one pt had no detectable disease upon pre-treatment restaging. The 7 pts who received JCAR021 had a median age of 63 years (range: 29 - 69). Both pts in the LMB ALL cohort had bulky extramedullary disease (> 5 cm diameter). One patient (LMB ALL cohort) had failed two allogeneic transplants and one patient (HMB ALL cohort) had failed an allogeneic transplant prior to treatment with JCAR021.
Four of 4 pts in the NHL cohort and 2 of 2 pts in the LMB ALL cohort received 7x105 JCAR021 cells/kg. The pt treated in the HMB ALL cohort received 7x104 JCAR021 cells/kg. No pt in any cohort developed grade ≥ 3 CRS. All ALL pts developed grade 2 CRS. No pts with NHL developed CRS; one pt in the NHL cohort who had CNS disease prior to CAR-T cell immunotherapy developed grade 3 neurotoxicity in the absence of CRS. We did not observe other neurologic events. No other grade ≥ 3 non-hematopoietic organ toxicity was observed and all 7 treated pts have completed response evaluation. Four weeks after infusion of a low dose of JCAR021, both patients in the LMB ALL cohort had undetectable marrow disease by high resolution flow cytometry and regression of bulky extramedullary disease (1 complete response [CR] and 1 partial response [PR] by PET-CT). One pt treated with a low dose (7x104 cells/kg) of JCAR021 in the HMB ALL cohort did not achieve CR (decrease in BM blasts from 79.8% to 29.5%) but CNS disease was cleared by flow cytometry. In the NHL cohort, we observed objective responses in 2 of 4 patients (1 CR, 1 PR). JCAR021 was detected in blood by flow cytometry and/or quantitative PCR for up to 112 days after infusion.
Conclusion
JCAR021 appears to have a favorable toxicity profile in R/R ALL and NHL pts. JCAR021 cells expanded in vivo and have persisted in all pts. We observed responses at very low doses of CAR-T cells in ALL pts with bulky disease. This trial continues to enroll to define optimal dosing and determine the safety and efficacy of JCAR021.
Hirayama:DAVA Oncology: Honoraria. Hay:DAVA Oncology: Honoraria. Till:Mustang Bio: Patents & Royalties, Research Funding. Kiem:Homology Medicine: Consultancy; Magenta: Consultancy; Rocket Pharmaceuticals: Consultancy. Shadman:TG Therapeutics: Research Funding; Mustang: Research Funding; Gilead: Research Funding; Pharmacyclics: Research Funding; AstraZeneca: Consultancy; Qilu Puget Sound Biotherapeutics: Consultancy; Acerta: Research Funding; Abbvie: Consultancy; Verastem: Consultancy; Genentech: Consultancy, Research Funding; Beigene: Research Funding; Celgene: Research Funding. Cassaday:Amgen: Consultancy, Research Funding; Seattle Genetics: Other: Spouse Employment, Research Funding; Adaptive Biotechnologies: Consultancy; Incyte: Research Funding; Pfizer: Consultancy, Research Funding; Merck: Research Funding; Kite Pharma: Research Funding; Jazz Pharmaceuticals: Consultancy. Acharya:Teva: Honoraria; Juno Therapeutics: Research Funding. Riddell:NOHLA: Consultancy; Adaptive Biotechnologies: Consultancy; Cell Medica: Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics: Equity Ownership, Patents & Royalties, Research Funding. Maloney:Juno Therapeutics: Research Funding; Seattle Genetics: Honoraria; Janssen Scientific Affairs: Honoraria; GlaxoSmithKline: Research Funding; Roche/Genentech: Honoraria. Turtle:Aptevo: Consultancy; Nektar Therapeutics: Consultancy, Research Funding; Caribou Biosciences: Consultancy; Gilead: Consultancy; Juno Therapeutics / Celgene: Consultancy, Patents & Royalties, Research Funding; Bluebird Bio: Consultancy; Eureka Therapeutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Consultancy; Precision Biosciences: Equity Ownership, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal